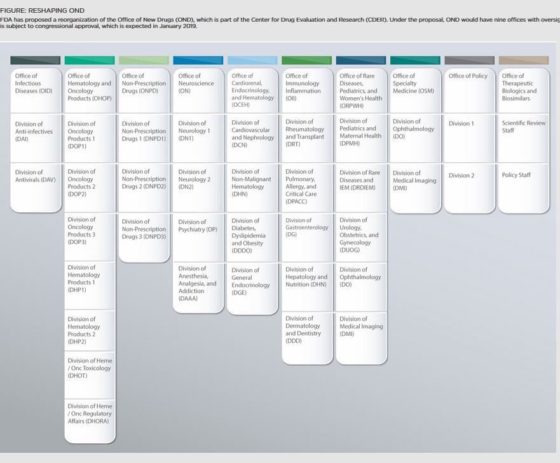
On June 4, 2018, FDA posted a statement from CDER Director Janet Woodcock announcing a multi-pronged FDA initiative to modernize FDA’s drug review offices and processes. The initiative will involve:
- Staffing increases
- Increasing the number of review offices from the current 5 to 9 and the review divisions from the current 19 to 30
- Establishing a multi-disciplinary review team at the outset of an application review, replacing the current system where the review division consults with other FDA offices as necessary during the course of a review
- Centralizing review procedures so that they are consistent across all review divisions, rather than having division-specific procedures, and concentrating administrative management within a group of regulatory experts
- Establishing a unified post-market safety surveillance system to monitor safety both pre- and post-approval
- Enhancing the patient’s voice in drug development
Click here for Dr. Woodcock’s statement, and here for a related statement from FDA Commissioner Gottlieb. Although this appears to be a significant internal initiative and reorganization, both statements contain very limited detail. There is only preliminary information on what the 30 review divisions will be, as captured in the illustration below from a tweet Dr. Gottlieb posted on Twitter (and even the illustration seems preliminary as the Division of Medical Imaging is listed twice). We’ll be monitoring this initiative and posting more information as we receive it.